Coronavirus Testing Workflows
Appleton Woods first priority in the fight against Covid-19 is to ensure that you have access to the equipment and consumables you need for testing and research.
To help with this we provide two different testing routes:
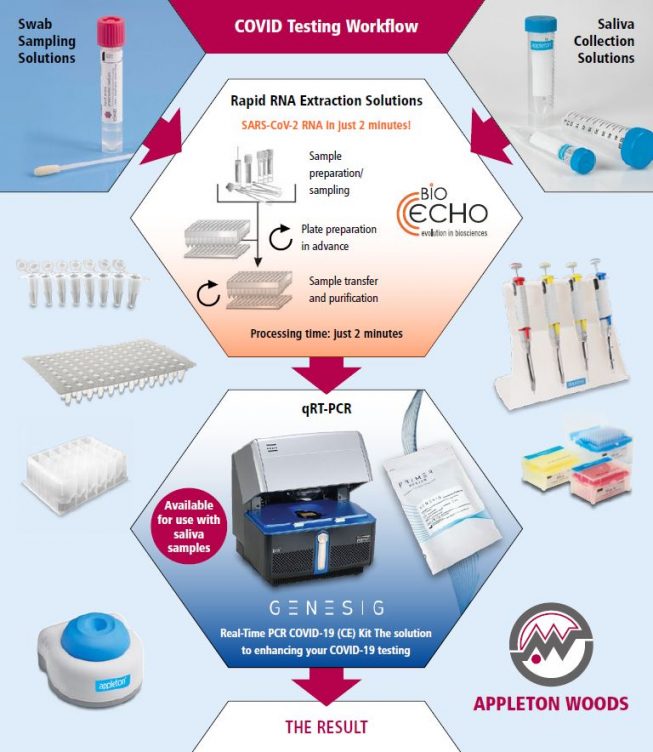
Rapid RNA Extraction Solutions
Primer Design genesig® Real-Time PCR assay
Complete Covid-19 Testing Package
For help with your requirements during these challenging times, please contact our Technical Team on 0121 458 7740, or email info@appletonwoods.co.uk. We’ll do our best to help.

Dr. Stewart Sale BSc (Hons), PhD
Product Specialist, Reagents
Email Stewart

Luke Housley BSc (Hons)
Key Account Manager
Product Specialist, Consumables
Email Luke
All-in-One Solutions
The Real-Time PCR diagnostic kit offers all-in-one multi-gene qualitative detection of SARS-CoV-2 ORF 1ab and nucleocapsid protein N gene.
Each bundle contains:
- Sansure IVD Kit, 48 tests (Part Number: PKIT13125DIAG)
- Sample Storage Reagent (X1002E)
- Sample Release Reagent (S1014E)
- Nucleic Acid Diagnostic Kit – Probes, Enzymes, Positive & Negative Controls (S3102E)
- Swabs
- 0.2mL PCR Tubes
We have put together a complete Covid-19 qPCR Testing Package, including:
- Swabs
- Pre-labelled tube with 1ml liquid medium and flocked swab
- Unique nylon flocked swab – a novel and patented design that ensures maximum sample recovery and sample release
- EchoLUTION viral RNA/DNA Swab Kit
- SARS-CoV-2 RNA extraction in just 2 minutes
- 96 swabs processed in less than 10 minutes
- Only standard lab equipment necessary
- COVID-19 genesig® Real-Time PCR assay
- Rapid detection
- High priming efficiency
- Accurate controls to confirm extraction, and assay validity
- Lyophilised components for ambient shipping
- CE-IVD marked and intended for in vitro diagnostic use in Europe
Please note that stock availability may be subject to change on certain lines. Contact us for the latest information on availability.
For our most recent Coronavirus Statement, please click here